|
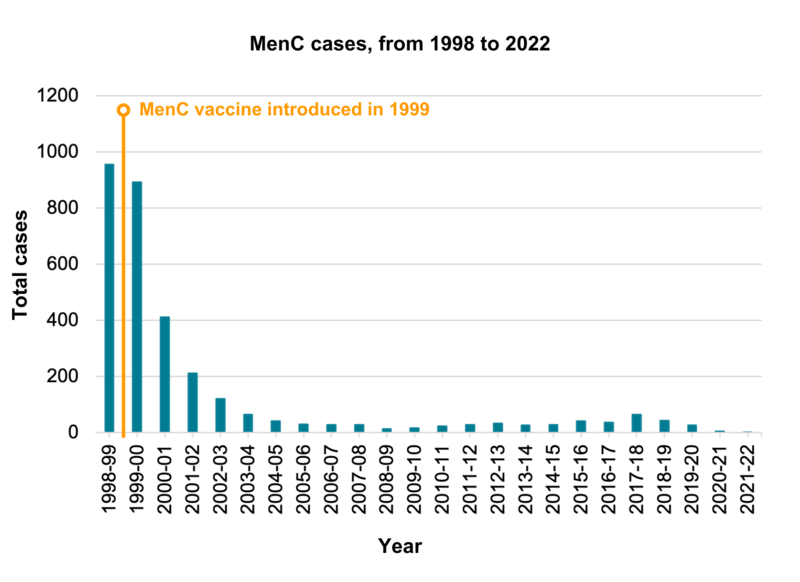
This vaccine gives protection against meningococcal disease, a major cause of meningitis, that is caused by group CNeisseria meningitidis bacteria. The MenC vaccination programme was introduced in the UK in 1999 and quickly led to a dramatic fall in the number of cases of MenC disease. Meningococcal bacteria are commonly carried in the back of the throat and passed around from person to person in an unvaccinated population. The vaccine prevents the carriage (the harbouring of) MenC bacteria in the back of the throat which means that even people who are not vaccinated are protected by herd immunity. The single vaccine programme ended in 2016. Instead, babies and children were protected through the Hib/MenC vaccine given at one year of age, and the MenACWY vaccine given to teenagers. However, the Hib/MenC vaccine for babies, Menitorix, is being discontinued by its manufacturer for commercial reasons. The UK government’s scientific advisory committee on vaccines, the Joint Committee on Vaccination and Immunisation (JCVI), has advised that MenC is now being controlled through the vaccination of adolescents, so babies born on 1 July 2024 onwards will no longer get MenC vaccine through the Hib/MenC. Currently, there are almost no cases of MenC disease in infants or young children in the UK. According to the UK Health Security Agency, there was just one reported in 2023/2024 in children aged 5 to 9, and two reported in adults over the age of 25. Before vaccination, there were nearly 1000 cases a year and 70-80 deaths a year – Public Health England and Health Protection Agency Archive.
|