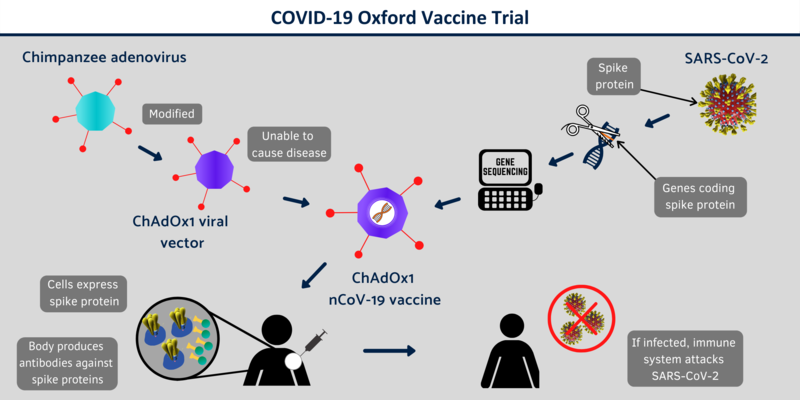
|
Most of the vaccines in the UK schedule are subunit vaccines which do not contain any whole bacteria or viruses at all. Instead, these vaccines typically contain one or more specific antigens (or “flags”) from the surface of the pathogen. The advantage of subunit vaccines over whole pathogen vaccines is that the immune response can focus on recognising a small number of antigen targets (“flags”).
Subunit vaccines do not always create such a strong or long-lasting immune response as live attenuated vaccines. They usually require repeated doses initially and subsequent booster doses in subsequent years. Adjuvants are often added to subunit vaccines. These are substances which help to strengthen and lengthen the immune response to the vaccine. As a result, common local reactions (such as a sore arm) may be more noticeable and frequent with these types of vaccines.
Recombinant Protein Vaccines
Recombinant vaccines are made using bacterial or yeast cells to manufacture the vaccine. A small piece of DNA is taken from the virus or bacterium against which we want to protect and inserted into the manufacturing cells. For example, to make the hepatitis B vaccine, part of the DNA from the hepatitis B virus is inserted into the DNA of yeast cells. These yeast cells are then able to produce one of the surface proteins from the hepatitis B virus, and this is purified and used as the active ingredient in the vaccine.
Most of the vaccines in the UK schedule are subunit vaccines which do not contain any whole bacteria or viruses at all. (‘Acellular’ means ‘not containing any whole cells’.) Instead these kind of vaccines contain polysaccharides (sugars) or proteins from the surface of bacteria or viruses. These polysaccharides or proteins are the parts that our immune system recognises as ‘foreign’, and they are referred to as antigens. Even though the vaccine might only contain a few out of the thousands of proteins in a bacterium, they are enough in themselves to trigger an immune response which can protect against the disease.
Recombinant vaccines used in the UK schedule:
Toxoid Vaccines
Some bacteria release toxins (poisonous proteins) when they attack the body, and it is the toxins rather than the bacteria itself that we want to be protected against. The immune system recognises these toxins in the same way that it recognises other antigens on the surface of the bacteria, and is able to mount an immune response to them. Some vaccines are made with inactivated versions of these toxins. They are called ‘toxoids’ because they look like toxins but are not poisonous. They trigger a strong immune response.
Toxoid vaccines used in the UK schedule:
Conjugate Vaccines
‘Conjugate’ means ‘connected’ or ‘joined’. With some bacteria, to get protection from a vaccine you need to train the immune system to respond to polysaccharides (complex sugars on the surface of bacteria) rather than proteins. But in the early days of polysaccharide vaccines it was found that they did not work well in babies and young children.
Researchers discovered that they worked much better if the polysaccharide was attached (conjugated) to something else that creates a strong immune response. In most conjugate vaccines, the polysaccharide is attached to diphtheria or tetanus toxoid protein (see ‘Toxoid vaccines’ above). The immune system recognises these proteins very easily and this helps to generate a stronger immune response to the polysaccharide.
On product information sheets the diphtheria toxoid is often called ‘CRM197 carrier protein’, because it is almost the same as diphtheria toxoid but not quite.
Conjugate vaccines used in the UK schedule:
- Hib vaccine (in the 6-in-1 vaccine and Hib/MenC vaccine), which contains a polysaccharide joined to tetanus toxoid
- MenC vaccine (in the Hib/MenC vaccine), which contains a polysaccharide joined to tetanus toxoid
- PCV (children’s pneumococcal vaccine), which contains polysaccharides from the surface of 13 types of the bacteria which causes pneumococcal disease joined to diphtheria toxoid (CRM197)
- MenACWY, which contains polysaccharides from the surface of four types of the bacteria which causes meningococcal disease joined to diphtheria or tetanus toxoid
There is also a conjugate vaccine for Typhoid fever, called Typhoid Conjugate Vaccine (TCV). This vaccine was shown to be effective in a study led by Oxford Vaccine Group, and is recommended by WHO to protect children from Typhoid fever in endemic regions such as Nepal and Bangladesh.
Virus Like Particles
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self-assemble into the virus-like structure. In some cases, the antigens in a VLP vaccine are the viral structural proteins themselves. Alternatively, the VLPs can be manufactured to present antigens from another pathogen on the surface, or even multiple pathogens at once. As each VLP has multiple copies of an antigen on its surface it is more effective at stimulating an immune response that a single copy. In some cases, the structural proteins of the VLP can act as adjuvants, helping to strengthen the immune response to the primary target antigen.
A handful of VLP-based vaccines are currently used worldwide:
OMV Vaccines
Outer Membrane Vesicles (OMVs) are naturally produced by bacteria and are essentially a bleb of the bacterial outer cell membrane. This contains many of the antigens found on the cell membrane but is a non-infectious particle. In the lab these OMVs can be harvested from bacteria to use as vaccines. The OMVs can also be modified so that toxic antigens are removed and antigens suitable for stimulating an immune response can be kept. OMVs also naturally act as adjuvants. This is a newer vaccine technology so there are few licenced examples:
|