Hib/MenC conjugate
The Hib/MenC vaccine is a conjugate vaccine (see our page on 'Types of vaccine'). This means that sugars (polysaccharides) are taken from the capsule around the Hib bacteria and joined to a non-toxic protein from tetanus.
The protein helps to stimulate the immune system in a broader way to respond well to the vaccine. This gives a better immune response in individuals of all ages.
How well does the vaccine work
The Hib/MenC vaccine was introduced in the UK in 2006, after studies showed that protection against Hib provided by the 5-in-1 vaccine (given at that time to babies at 8, 12 and 16 weeks) waned (decreased) during the second year of life.
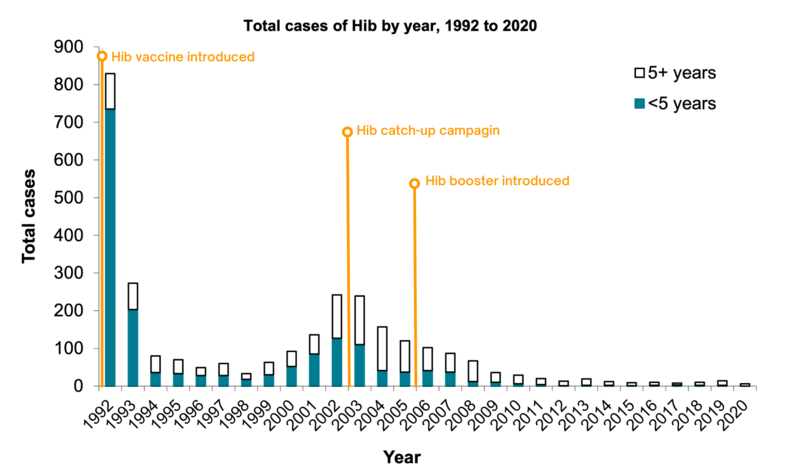
In 1991, the year before a Hib vaccine was introduced, there were 759 confirmed cases of invasive Hib in children under five in England. In 2020, there were no cases in children under 5 years of age eligible for immunisation.
Source: UK Health Security Agency
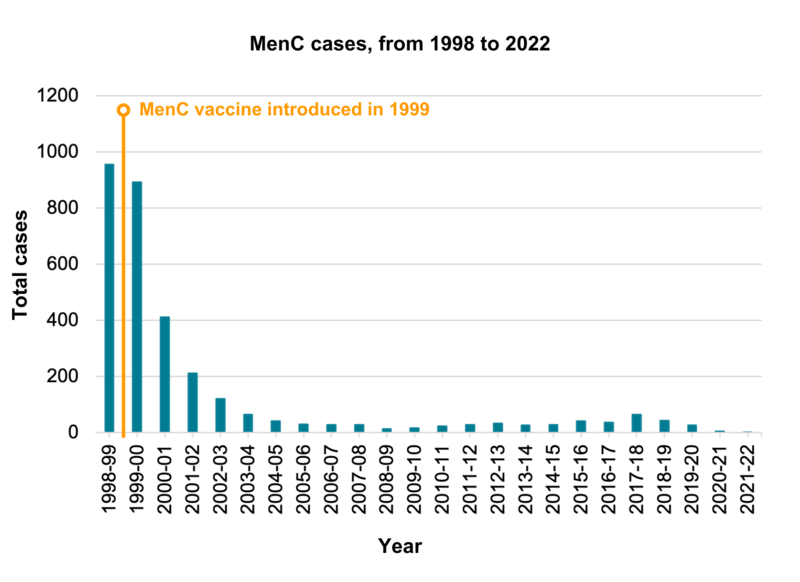
Following the introduction of the MenC vaccine in 1999, the number of cases of meningococcal disease caused by group C bacteria fell by over 90% in vaccinated groups. There was a very slight rise in cases since 2014-15. MenC cases then continued to fall, and remain low, with 27 cases in 2019-2020, five cases in 2020-2021, and one case reported in 2021 to 2022.
Click here for an accessible text version of this graph
Sources: Public Health England , UK Health Security Agency and the Health Protection Agency Archive 
JCVI advice
The Joint Committee on Vaccination and Immunisation (JCVI) is an expert scientific advisory committee that advises the UK government on vaccination and immunisation.
The JCVI has been notified that Menitorix, the Hib/MenC currently used in the UK, will be discontinued by the manufacturer for commercial reasons.
As Menitorix is the only Hib/MenC combination product currently available in the UK, changes to the routine infant schedule are necessary. It is estimated that, based on current UK stocks of Menitorix, the current routine schedule can continue until 2025.
Once current stocks run out, the JCVI advises a change to the immunisation schedule to maintain protection of young children with alternative products:
- an additional dose of Hib-containing multivalent (containing multiple strains) vaccine should be offered at 12 or 18 months of age – note that giving this at 18 months would require the creation of a new immunisation visit
- the second dose of measles, mumps and rubella (MMR) vaccine should be brought forward from 3 years 4 months to 18 months of age to improve coverage. The JCVI has also recommended that the vaccine for chickenpox should be added to the schedule at 12 and 18 months of age using the combined MMRV (measles, mumps, rubella and varicella) vaccine.
- based on the demonstrated decline of invasive meningococcal A, C, W and Y disease in the UK (primarily due to the success of the teenage MenACWY vaccination programme) and the subsequent low number of cases to prevent, the inclusion of a MenC-containing vaccine (such as MenACWY) in the infant schedule is not recommended. Efforts to sustain and improve coverage of MenACWY in adolescents are important to maintain herd immunity.
See the JCVI statement here
See the JCVI chickenpox statement here